Medical Device User Fee Rates for Fiscal Year 2023
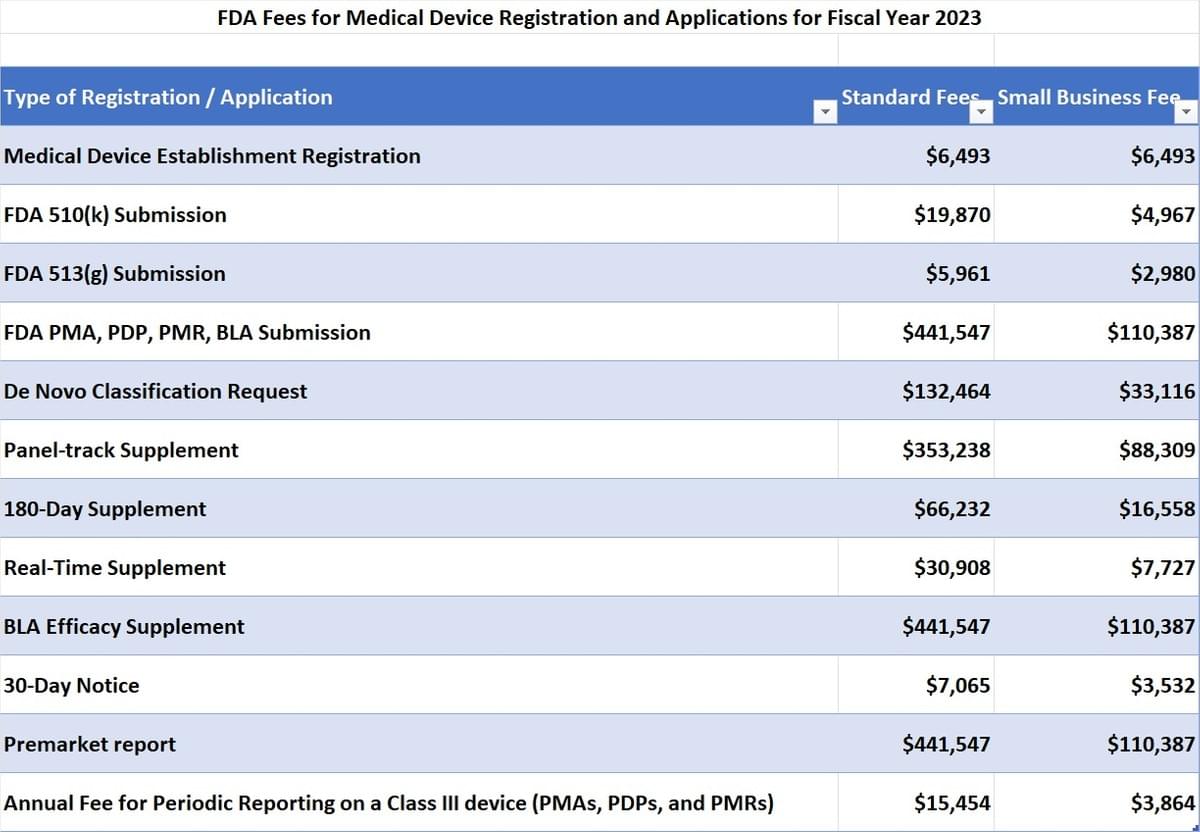
Owners or operators of establishments that are involved in the production and distribution of medical devices intended for use in the U.S. are required to register annually with the FDA. This process is known as establishment registration (Title 21 CFR Part 807).
Congress has authorized the FDA to collect an annual establishment registration fee for device establishments. A detailed list of the types of device establishments that are required to register and pay the fee can be found at "Who Must Register, List and Pay the Fee." There are no waivers or reductions for small establishments, businesses, or groups.
The annual registration user fee for fiscal year 2023 follows:
YearFeeFY 2023$6,493
